研究人员为燃料电池开发了低成本,更高效的纳米结构
移动用户编辑短信CD到106580009009,即可免费订阅30天中国日报双语手机报。
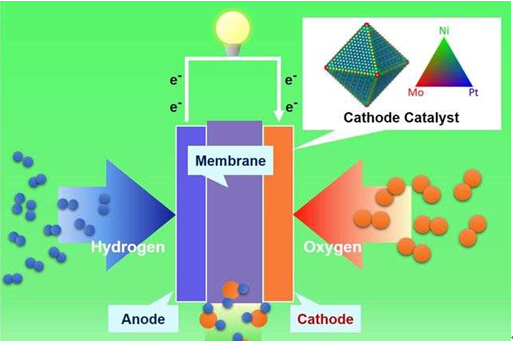
中国科技网6月16日报道(张微编译)加州大学洛杉矶分校亨利·萨姆厄里工程与应用科学学院的研究人员领导一个研究团队,开发出使用三种金属化合物制成的纳米结构,在降低生产成本的同时,增加了燃料电池的效率和耐久性。他们的方案解决了这项技术一直停滞不前的棘手问题。
加州大学洛杉矶分校材料科学与工程专业副教授,这项研究的首席研究员Yu Huang,将研究成果发表在6月12日的《科学》期刊上。
质子交换膜燃料电池作为清洁能源技术,有着广泛的应用包括在零排放汽车上的使用。燃料电池的工作原理是引发氢燃料和空气中的氧气发生化学反应产生电力,而且它们产生的副产品是水而不是传统汽车排放的污染物和温室气体。
发生在质子交换膜燃料电池中的化学反应是由金属催化的。这些化学反应中有一个是氧化还原反应,它通常使用的铂作为催化剂。但铂的高成本一直是阻碍广泛采用燃料电池的主要因素。科学家们研究了替代催化剂包括用铂–镍化合物,但到目前为止,没有得到一个可行的解决方案。
研究人员使用了一种被称为“表面掺杂”的表面工程技术,发明了一个更高效,更持久以及生产成本更低的燃料电池,他们在电池中铂镍纳米结构的表面加入了叫做钼的第三种金属。这个变化使合金表面更稳定而且能够防止长时间使用过程中镍和铂的损失。
这项研究发现,铂镍钼表面的纳米结构比目前市场上的铂碳复合催化剂效率高出81倍。而且这个三种金属化合物使用一段时间之后,仍能保持95%的催化效率,明显优于铂镍催化剂66%或更少的催化效率。
“我们发现第三种过渡金属的加入,明显提高了效率和耐久性,降低了成本,”Huang说,他也是加利福尼亚纳米技术研究院的成员。“此外,表明掺杂技术也可以应用于一系列的催化剂中,同时为环境保护,能源生产和化工产品寻找高效催化剂的催化剂工程开辟了一条新路径。”
Researchers develop lower-cost, more efficient nanostructure for fuel cells
A team led by researchers at the UCLA Henry Samueli School of Engineering and Applied Science has developed nanostructures made from a compound of three metals that increases the efficiency and durability of fuel cells while lowering the cost to produce them. Their solution addresses vexing problems that have stalled the adoption of this technology.
Yu Huang, a UCLA associate professor of materials science and engineering, was the principal investigator of the research, which was published in the June 12 issue of Science.
Proton exchange membrane fuel cells have shown great promise as a clean energy technology with numerous applications including zero-emission vehicles. The fuel cells work by causing hydrogen fuel and oxygen from the air to react to produce electricity, and the exhaust they create is water—rather than the pollutants and greenhouse gases emitted by traditional car engines.
The chemical processes that take place in proton exchange membrane fuel cells are catalyzed by metals. One of those processes is an oxygen reduction reaction, which has typically used platinum as its catalyst. But the high cost of platinum has been a major factor in hindering wider adoption of fuel cells. Scientists have studied alternative catalysts—including using a platinum–nickel compound—but to date, none has been durable enough to be a viable solution.
To create a fuel cell that would be more efficient, more durable and less expensive to produce, the researchers used a surface engineering technique called "surface doping," in which they added a third metal called molybdenum to the surface of platinum-nickel nanostructures. The change made the alloy surface more stable and prevented the loss of nickel and platinum over time.
The study found that nanostructures with the platinum-nickel-molybdenum surface were 81 times more efficient catalysts than catalysts made from a commercial platinum-carbon compound. And the three-metal compound retained about 95 percent of its efficiency over time—significantly better than the efficiency rate of 66 percent or less for platinum-nickel catalysts.
"We showed that the addition of a third transition metal enables improvement in both efficiency and durability to bring down long-term costs," said Huang, who is also a member of the California NanoSystems Institute. "In addition, the surface doping approach may also apply to a broad range of catalysts and opens up a new route for catalyst engineering for the search of high performance catalysts for environment protection, energy generation and chemical productions."
热门推荐
更多> 中国日报一周图片精选:6月27日-7月3日
中国日报一周图片精选:6月27日-7月3日  3D打印服装高跟鞋亮相软博会
3D打印服装高跟鞋亮相软博会  浙大美女硕士肤白貌美大长腿
浙大美女硕士肤白貌美大长腿
 藏北牧人达瓦一家的故事
藏北牧人达瓦一家的故事  拉萨捎信:西藏县县通柏油路即将实现
拉萨捎信:西藏县县通柏油路即将实现  强降雨致江西武宁突发山洪
强降雨致江西武宁突发山洪 吉林载28人大巴冲出大桥 10名韩国人身亡(图)
吉林载28人大巴冲出大桥 10名韩国人身亡(图)  2015年铁路暑运今日拉开帷幕
2015年铁路暑运今日拉开帷幕  两只大熊猫落户长春 与游客见面
两只大熊猫落户长春 与游客见面
 中国特技滑水队秀四川青神上演"空中飞人"(高清组图)
中国特技滑水队秀四川青神上演"空中飞人"(高清组图)  图片精选:7月1日全国铁路实行新运行图
图片精选:7月1日全国铁路实行新运行图  7月1日全国铁路实行新运行图
7月1日全国铁路实行新运行图 北京雾霾散去 迎蓝天白云
北京雾霾散去 迎蓝天白云  北京大红门桥附近起火
北京大红门桥附近起火  台湾粉尘爆炸事件一伤者转院上海救治
台湾粉尘爆炸事件一伤者转院上海救治
 西安小伙毒驾北大街被查 三天前才染上K粉
西安小伙毒驾北大街被查 三天前才染上K粉  解放军夜训大片:战车火炮怒吼 彩光炫目
解放军夜训大片:战车火炮怒吼 彩光炫目  高清:江苏电力防汛应急 抢修人员乘艇抢险
高清:江苏电力防汛应急 抢修人员乘艇抢险